Sodium bicarbonate, also known as baking soda, is often used by people living a zero-waste lifestyle, especially when doing the laundry or the housework. One might understandably wonder what it actually is. Where does it come from? How is it produced? Discover all the secrets of baking soda in this article.
TABLE OF CONTENTS
1. What is sodium bicarbonate?
Chemistry
Not to be confused
2. A brief history of baking soda
3. Production of baking soda
4. The properties of baking soda
5. In conclusion...
1. What is sodium bicarbonate?
From a chemical perspective
Sodium bicarbonate is an inorganic compound which has the formula NaHCO3. Sodium bicarbonate is available in the form of white powder. It is neither corrosive nor irritating, so no special measures should be taken when handling it.
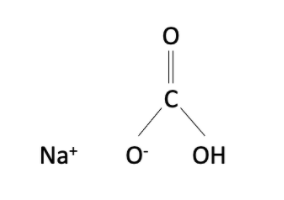
Not to be confused
Be careful not to confuse bicarbonate with caustic soda, soda crystals or percarbonate.
Caustic soda, or sodium hydroxide, is a strong base with the formula NaOH. It should not be used like bicarbonate because it is very corrosive (it can cause severe burns and irritation), so you should be very careful.
Soda crystals, or sodium carbonate, has the empirical formula Na2CO3. Soda crystals are more irritating than sodium bicarbonate but less so than caustic soda. Nevertheless, one should pay attention and should preferably handle the crystals with gloves.
Sodium percarbonate has the empirical formula 2 Na2CO3-3H2O2. It is also an irritant, so take care. It is mainly used to bleach fabrics.
2. A brief history of baking soda
Baking soda is nothing new... The ancient Egyptians already used a kind of baking soda, called natron. It is a mixture of carbonate and bicarbonate of soda that was found in some lakes.
The Egyptians used bicarbonate to wash themselves and to brush their teeth.

It was much later, in 1797, that the Leblanc process enabled the industrial manufacture of sodium bicarbonate for more than half a century. This process was dethroned by the Solvay process.
3. Production of baking soda
Sodium bicarbonate may therefore be found naturally in some lakes, but this is not how most of it is produced today.
In 1863, Ernest Solvay, a Belgian chemist, started the industrial production of sodium bicarbonate. This reaction is based on salt (NaCl) and chalk (CaCO3) with a large number of intermediate products.
2 NaCl + CaCO3 → CaCl2 + 2 Na2CO3
Na2CO3 + H2O + CO2 → 2 NaHCO3
The advantage of this process is that it is made from two very cheap and abundant products: salt and chalk. It has allowed the development of industries such as glass manufacturing, which needs sodium bicarbonate.

4. The properties of baking soda
Baking soda is alkaline, which means it reacts well with acids. This property is very interesting because most bad smells are caused by acids. It is therefore a very good deodorizer.
Baking soda also has an abrasive texture that is interesting for cleaning. This is one of the reasons why it is used a lot in this field.
Bicarbonate dilutes easily in water, which gives it the advantage of being easily used for cleaning in combination with other products, such as vinegar.
Note that baking soda is biodegradable. It is neither toxic for our health nor for our environment!
If you want to know more about the uses of bicarbonate, don't hesitate to visit our other blogs on this subject.

5. To conclude ...
Baking soda is very cheap and easy to use in many ways. Discover it and get rid of a lot of expensive products.
See you soon!
Titi the Wapiti
